Get our independent lab tests, expert reviews and honest advice.
Philips Respironics recall puts patients in a bind

Need to know
- Clinical information for people with sleep apnoea and other conditions has been confusing and conflicting
- Philips is focusing on repairs rather than refunds, but that process could take many months
- Patients now face a ‘horrible decision’, says one sleep researcher
The July recall of 14 sleep and respiratory care devices by Philips Electronics Australia has left patients uncertain about when their machines will be fixed and unclear about whether they should keep using them.
The product defect is a polyurethane foam component that can degrade into particles and be ingested or inhaled by the user. Whether or not it degrades, the foam emits potentially dangerous chemicals with no known tolerance levels.
(The recalled products are Continuous Positive Airway Pressure (CPAP) and Bi-Level Positive Airway Pressure (Bi-Level PAP) devices and mechanical ventilators. See the TGA recall notice for a full list).
Philips is unable to confirm the repair or replacement timeframes
Pharmacies have been directed to take the machines off the shelves, but patients have been told to keep on using them until they can get an appointment with their doctor and further advice.
At the same time, the company says, “the risks identified have resulted in Philips recommending discontinued use” of the devices, with the exception of life-sustaining mechanical ventilators.
And there’s no word on when the issue will be resolved. “Due to the volume of devices, we regret it may take some time to repair or replace your device,” the company says. “Presently, Philips is unable to confirm the repair or replacement timeframes.”

‘Stuck with a horrible decision’
A sleep researcher at a major Australian University says the recall has left its customers in a tight spot.
“It has posed significant issues for many people,” the researcher says. “The recall is clearly slow, and it’s a bit challenging for people who are using or have been using one of their devices and all of a sudden they’re told there’s a potential health risk if you continue using it.
“But there’s a health risk if they don’t use it as well. They’re really stuck with a horrible decision to make.”
Seeing a doctor
The advice from Philips and the TGA that patients should continue using the devices until they speak to their doctors is also problematic, the researcher says.
“There are hundreds of thousands of people in Australia who are using these devices, and if they’re all trying to get in to see their physicians that’s a huge impost on the healthcare system.”
On 18 August, more than six weeks after the recall came into effect, the TGA added a section to its ‘product defect correction’ notice saying Philips “is waiting for stock of replacement foam to arrive from overseas”, which it expects in September for the DreamStation platform, covering just four products on the recall list.
The update also says the company is “responding to consumer requests for refunds”.
Poor communication from the start
Chris* suffers from severe sleep apnoea and has been relying on his Philips CPAP machine to help him keep breathing through the night for the past three years. (*Not his real name.)
“I was watching A Current Affair,” Chris says. “That’s how I found out there was an issue.”
The program featured a Philips device user who had unknowingly inhaled particles from somewhere within the machine, which he discovered after blowing his nose.
“Their communication has been very poor,” Chris says. “They’ve known about this problem since April worldwide, and it’s only on the sixth of July that they did the official recall in conjunction with the TGA.”
Philips has our email addresses. They could have told people, but they didn’t
(Philips Australia’s parent company Koninklijke Philips, based in the Netherlands, announced the recall on June 14, although it acknowledged the problem in an April 26 report.)
Chris regularly checks a Philips website that’s linked to his unit to monitor the quality of his sleep, but there was no message about a recall there.
“They can message us, and I’m really disappointed that they didn’t,” Chris says of Philips customers in his predicament. “Philips has our email addresses. They could have told people, but they didn’t.”
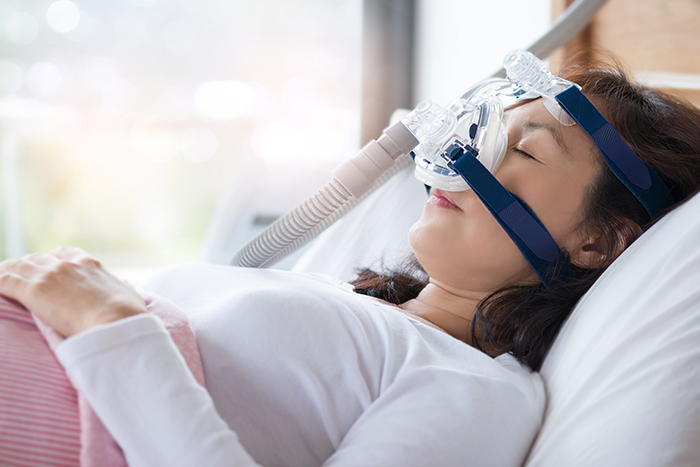
Contacting customers ‘where possible’
The TGA says Philips is in the process of contacting everyone who has registered their device. Yet the Philips recall notice is less committal, saying the company will contact customers “where possible”.
Both the Philips and TGA recall notices instruct patients to register their devices on the Philips website, but that process has been far from smooth.
We asked how many defective devices it has sold in Australia, but Philips wouldn’t give us an answer
Customers who did hear about the recall have had trouble getting in touch with Philips. And customers who were able to register their devices didn’t initially receive any confirmation that they’d done so, although Philips has now apparently corrected this.
We asked Philips if customers have to register their device in order to be contacted, but the company didn’t respond to this question.
No timeline for repair or replace
We also asked the company how many defective devices it has sold in Australia, but it wouldn’t give us an answer, saying “Philips does not disclose installed base information”. (Philips Electronics Australia, a market leader, posted revenues of about $574 million in 2020.)
And we asked for a rough estimate of the repair or replace timeframe and whether it could take as long as 12 months, for instance, but Philips didn’t respond to that question either.
Users of the devices have also been told not to attempt to remove the foam themselves, but Chris says he’s seen YouTube videos of people doing just that.
Corporate messaging from Philips
For patients using life-sustaining mechanical ventilators, the advice is to keep using the machine and make an appointment with your doctor for further advice.
“Stopping treatment suddenly could have an immediate and detrimental effect on your health,” the TGA says.
Chris agrees. In the sleep study test that led to his sleep apnoea diagnosis, Chris’s breathing stopped 86 times an hour and his blood oxygen dropped as low as 70%. “If I stop using the machine, it’s very dangerous,” Chris says.
If I stop using the machine, it’s very dangerous
Philips admits that mechanical ventilator patients have little choice but to keep using their machines regardless of any medical advice. The company says it “recognises that alternate ventilator options for therapy may not exist or may be severely limited for patients who require a ventilator for life-sustaining therapy”.
For patients who need to keep using the machines, Philips recommends installing an inline bacterial filter.
No refunds on offer?
With no response from Philips and no timeline for repair, Chris called the company on July 8 looking for a refund for the $1300 unit.
“They said that my case would be escalated,” Chris says. But nothing happened.
Chris rang Philips again on July 19 to say he still hadn’t received email confirmation that his device had been registered. The Philips staff member told him over the phone it had..
On July 23, nearly three weeks after Philips issued the recall, Chris finally got an email from the retailer that sold him the machine. The retailer told him to talk to Philips if he wanted a refund. (Under Australian Consumer Law, or ACL, retailers are obligated to give refunds in the case of a major fault and can’t send you back to the manufacturer.)
Our priority is to replace the foam with the new material in all the affected devices either by repairing or replacing with like devices
Philips spokesperson
In its message to customers, Philips emphasises that it aims to repair or replace the devices. The company makes reference to customers’ rights under the ACL but stops short of using the word ‘refund’.
We asked Philips if it was offering refunds, but a company spokesperson stressed that “our priority is to replace the foam with the new material in all the affected devices either by repairing or replacing with like devices” – although the spokesperson did add that “consumers’ rights under the Australian Consumer Law will always be in addition to any remedy that Philips may provide”.
12 months to replace
Alan Young, president of the Australasian Sleep Association and a sleep disorders and respiratory physician, tells CHOICE the organisation supports the TGA recommendation that people continue using the potentially faulty devices until they talk to their doctors, saying “the TGA has clearly reviewed the safety data”.
“Every case needs to be assessed on its own merits about the risks and benefits of continuing treatment until the machines are replaced,” Young says.
“There have been no reports of deaths or long-term harmful effects, but because there’s a chemical compound involved there’s always a risk of toxic or carcinogenic effects longer term,” Young says, stressing that there’s no current data to support this concern.
One of the compounds linked to fatal poisonings
One of the compounds, diethylene glycol (DEG), is used in a wide range of industrial products including antifreeze and brake fluid, and has been linked to fatal poisonings.
Toothpaste containing more than 0.25 per cent of diethylene glycol by weight is banned in Australia. According to the ACCC, “medium to long-term exposure to DEG at significant levels may create unacceptable health risks”.
It’s going to be a huge logistics exercise for Philips to do this and it’s going to take many months to complete that process
President of the Australasian Sleep Association Alan Young
Another chemical compound in the foam, toluene diisocyanate, is classified as toxic by inhalation and potentially fatal if breathed in.
According to the TGA, Philips is investigating whether the volume of compounds would exceed “tolerable intake” levels. The spokesperson told us “the company continues to review potential health risks related to this issue”.
Young says Philips data reviewed by the TGA indicates that three in 10,000 users have reported side effects. He acknowledges that the repair and replace operation will be a tall order for Philips.
“To replace all of these devices, it could take as long as 12 months,” Young says. “It’s going to be a huge logistics exercise for Philips to do this and it’s going to take many months to complete that process.”
The Australasian Sleep Association asked Philips whether the company would cover the costs of hiring a machine before repairs or replacements are available, but had yet to receive a response when we spoke to Young in mid-August.
Corrective action ‘as soon as possible’
Chris has installed inline bacterial filters so that he can keep using the machine, but he’s worried about the chemicals.
No-one knows about the cumulative effect of these volatiles going up my respiratory tract
“Philips says no-one had died from this issue,” he says. “Well that’s very nice of them. But no-one knows about the cumulative effect of these volatiles [chemical compounds] going up my respiratory tract. The inline filters do not stop the volatiles.”
He contacted his doctor and got a call back a week later saying that, given the severity of his sleep apnoea, he should continue using the machine. Shortly after, he bought a new one.
“I’m not going to entertain the notion of replacing or repairing the foam,” Chris says.





